
a future free from
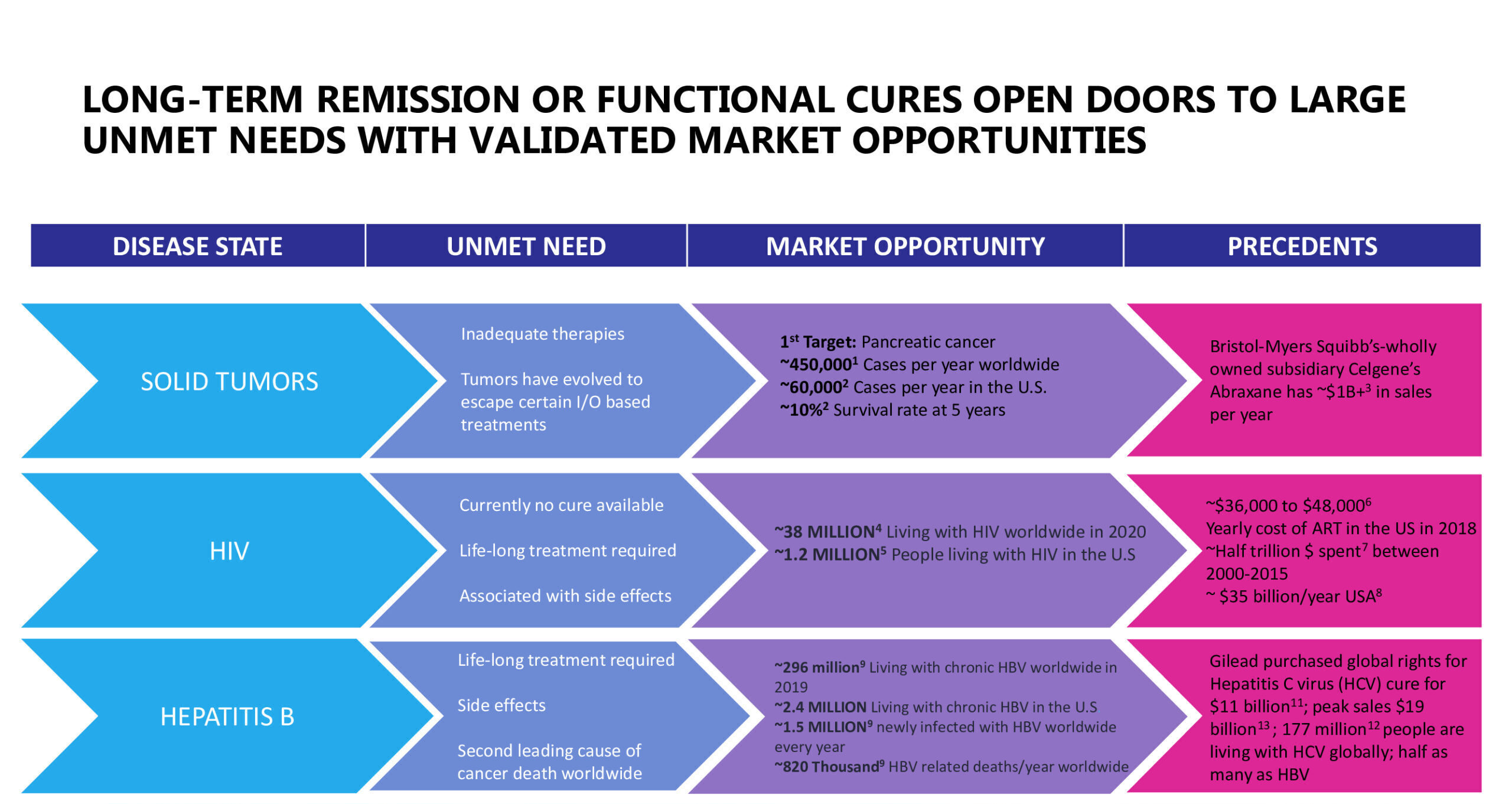
Market Opportunities
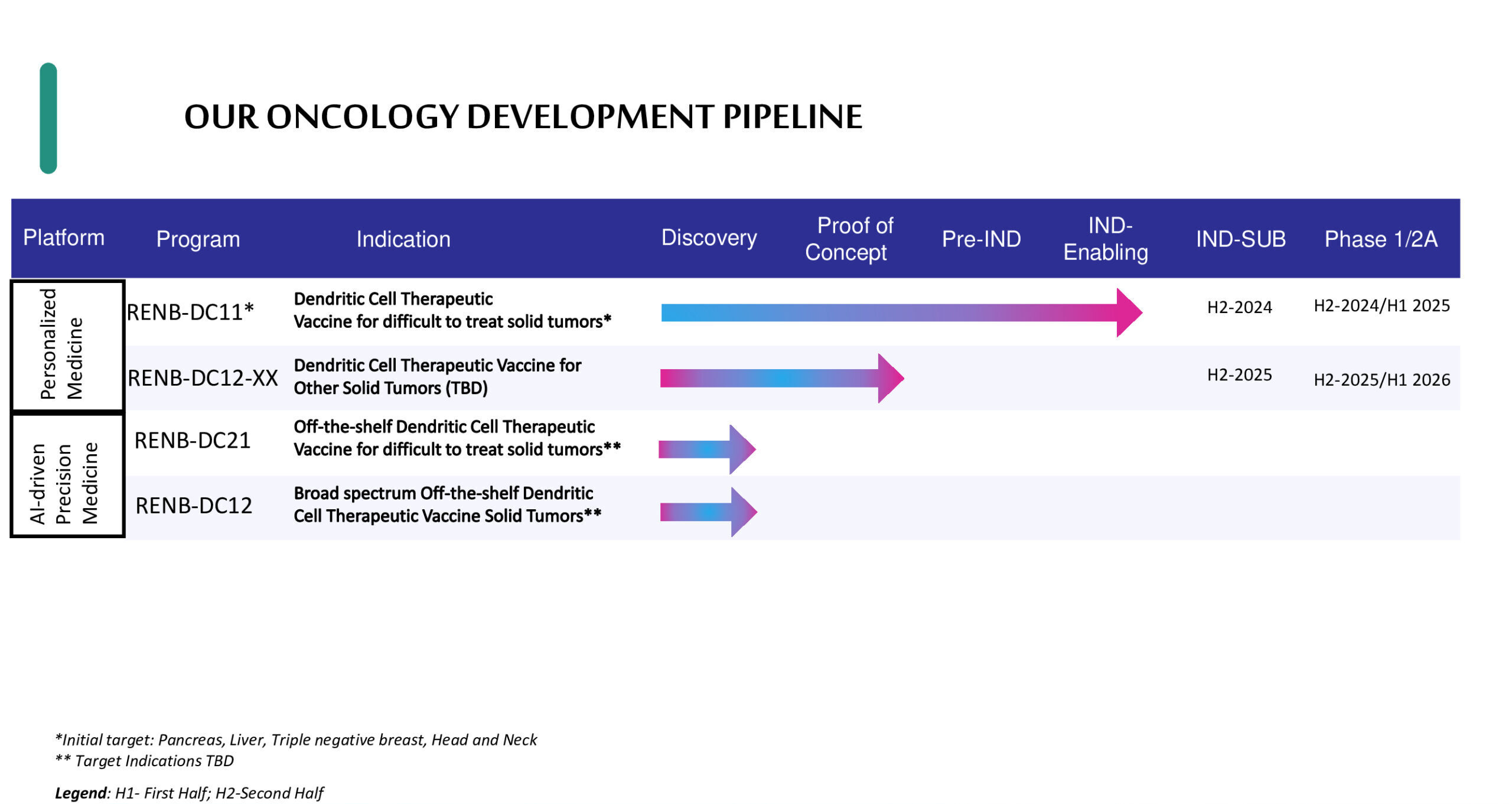
Pipeline and Technology Platforms
Our integrated platforms encompass innovative interventions in advanced cell and gene therapies that provide hope for cures and/or life-long remissions for devastating diseases. Because of the relative ease of administration, our potentially ground-breaking interventions could be used throughout the world to transform the lives of millions of people.
Oncology Platform
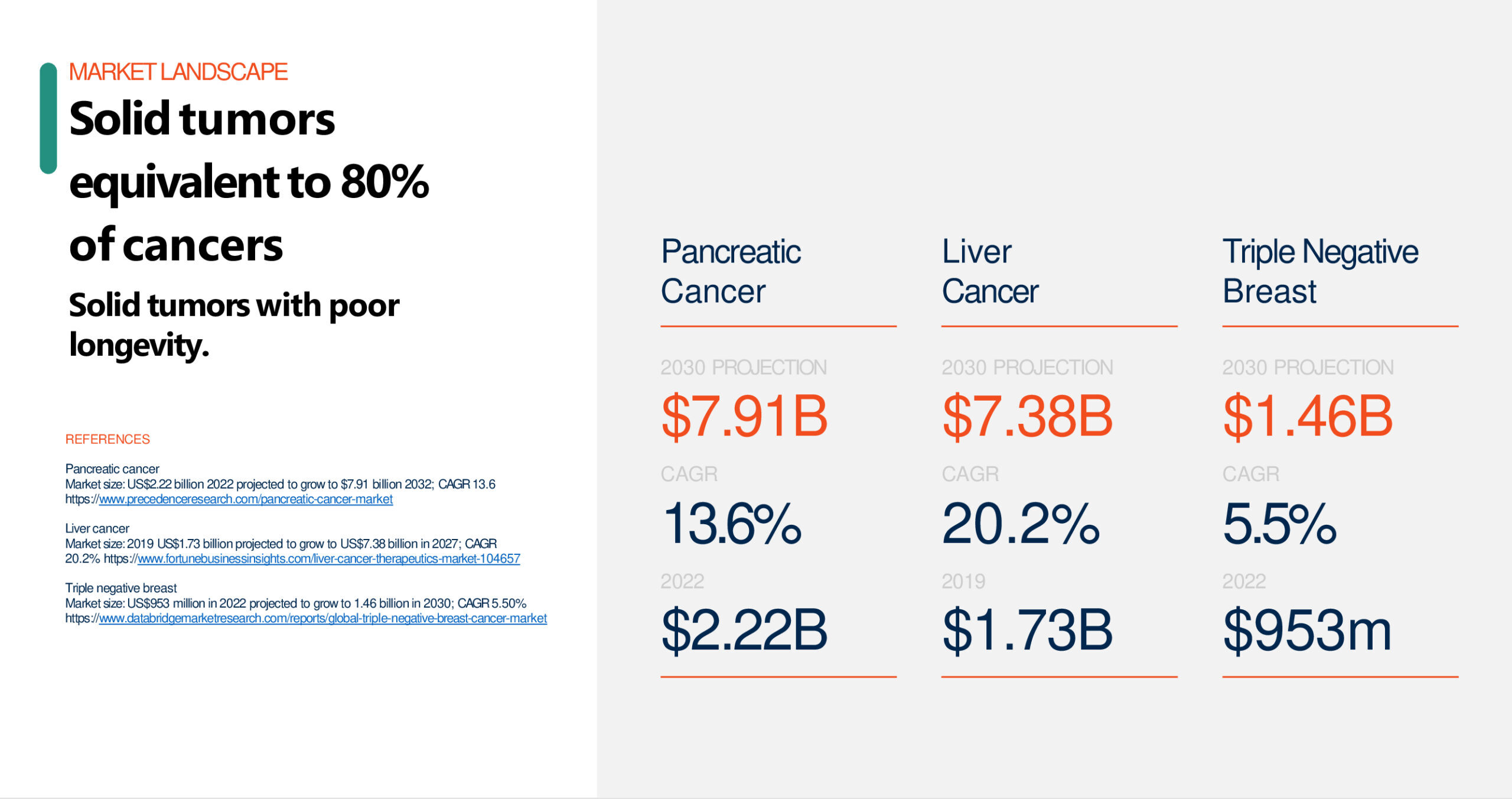
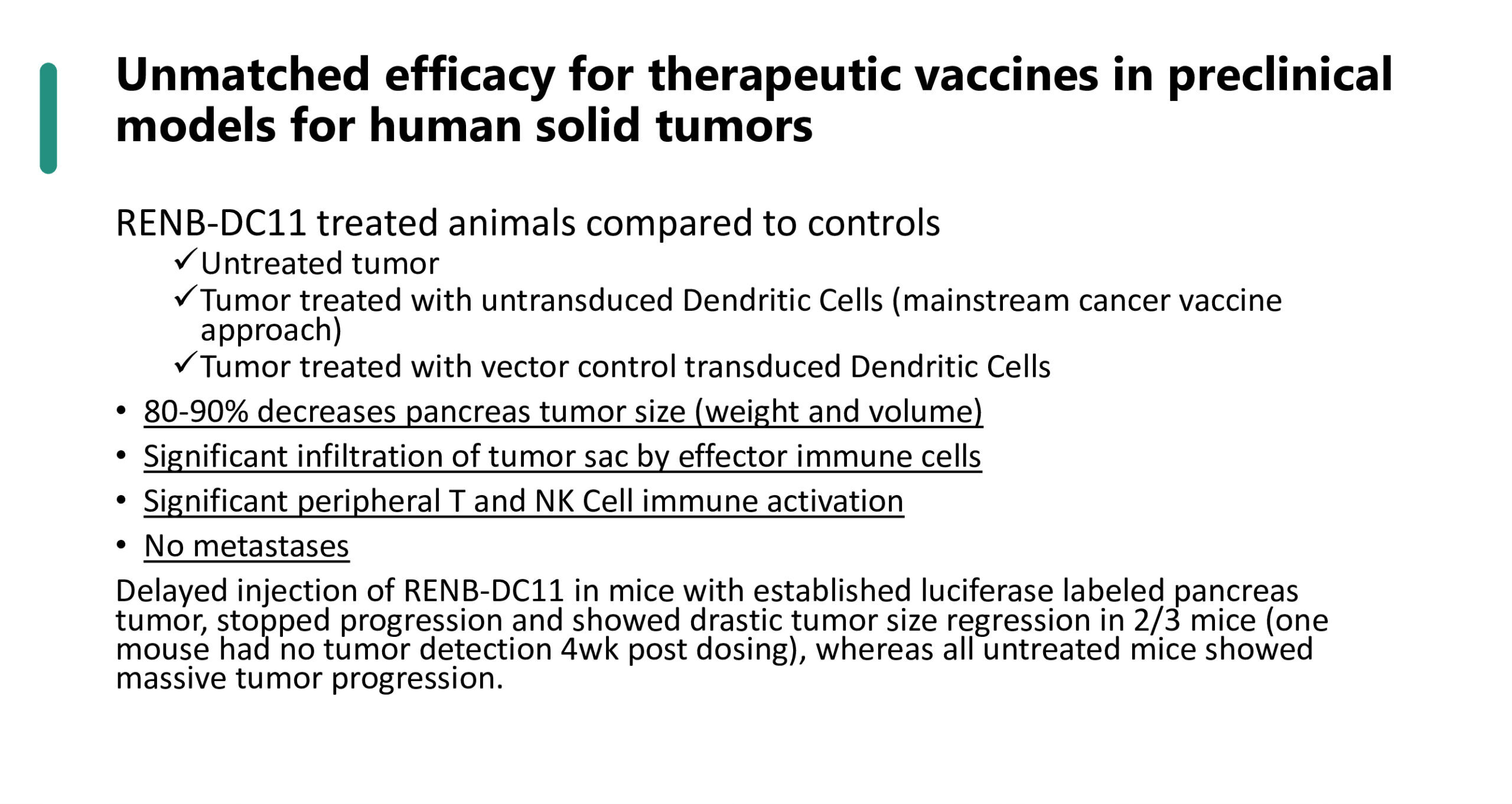
Gene Modified Cell Therapies for Cancer / Solid Tumors
Currently into IND-enabling phase with planned IND submission H2 2024
Cancer cells can evolve to evade immune responses by releasing inhibitory signals against, or hiding from, key parts of the immune system designed to seek and kill tumors. Solid tumors, unlike cancer of the blood (lymphomas), have shown particular resistance to even the most advanced immune-therapies.
Renovaro Biosciences is developing a novel platform of therapeutic cancer vaccines using allogenic dendritic cells that are genetically engineered by design to bypass tumor immune response evasion and enhance tumor eradication. Dendritic cells are the professional antigen presenting cells in the body. Their role is to patrol our body, capture pathogens and to educate our immune system to recognize and eliminate unwanted cells and pathogens.
RENB-DC11: Using a proprietary combination of genetic modifications with known activity to enhance immune signaling, we have tailored a new and improved version of dendritic cells that have shown remarkable ability to eliminate aggressive forms of human solid tumors in preclinical models.


Leveraging banked (off-the-shelf) allogenic cell therapy
Utilizing allogenic cells had several advantages to our development strategy.
1. Functional activation of immunity: Non-self allogenic cells further T-Cells.
2. Possible enhanced safety: allogenic cells will be rejected naturally after they performed their role in activating cancer killing cells, and will not permanently reside in the body.
3. Faster access to therapy after diagnostic. Large cell bank of cryopreserved dendritic cells that are ready to be pulsed with patient tumor can decrease time between diagnostic and treatment. Moreover, our ability to scale up production using a single donor source of stem cells will provide a more cost effective solution than autologous cell therapy.
4. True platform of “ready to be loaded” with any tumor antigen allows for faster development of multiple products addressing multiple cancer types.
Renovaro BioSciences has initiated a collaboration with Dr. Anahid Jewett from UCLA to study further the in vitro and in vivo effectiveness of the approach in pancreatic cancer. Dr. Jewett created an innovative pancreatic cancer mouse model that mimics the human immune system in combination with implanted human cancer cells…
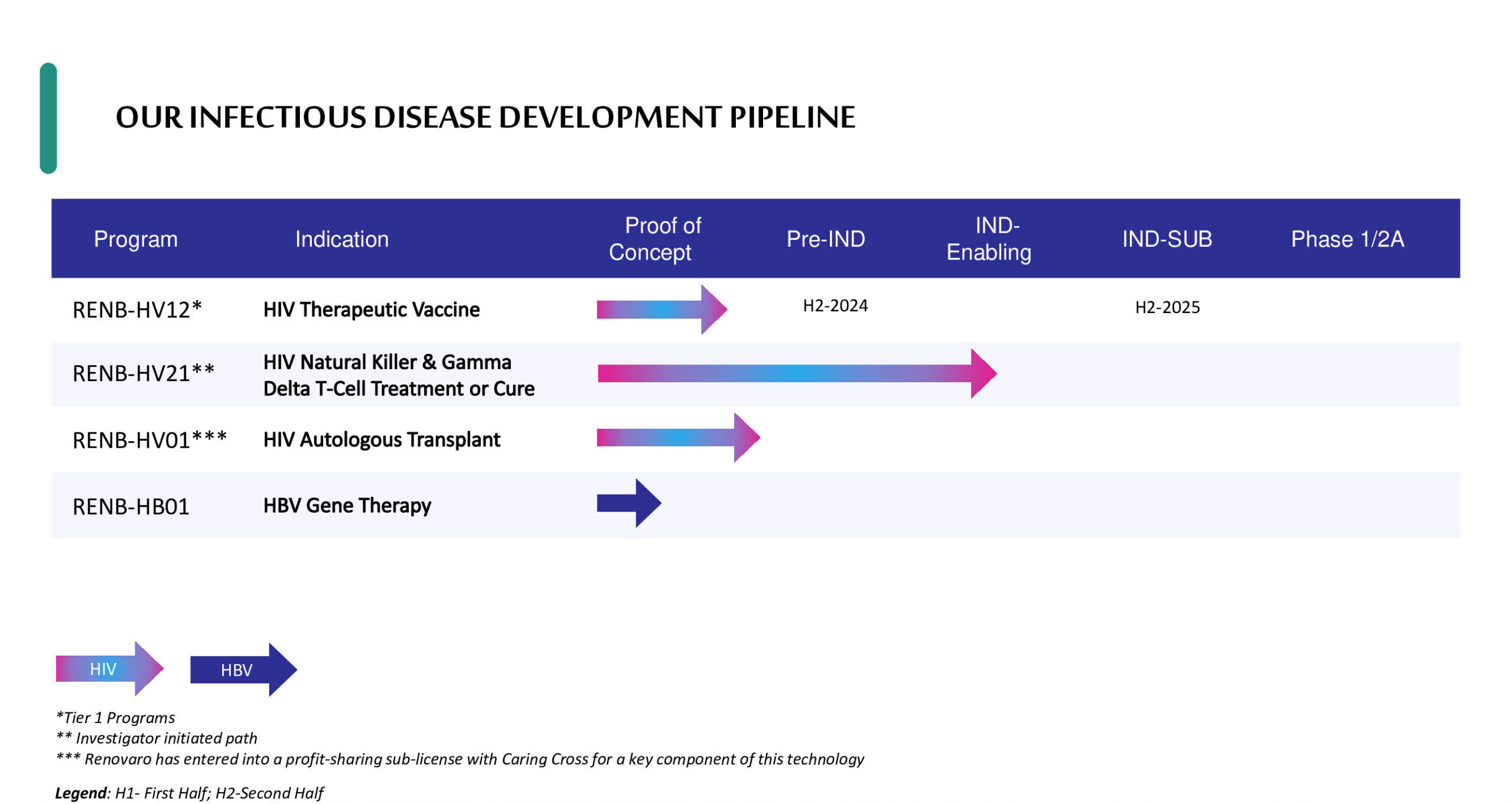
infectious diseases platform

Gene Modified Cell Therapies for HIV
HIV Overview
Antiretroviral therapy (ART) has changed HIV-infection from a death sentence to a chronic disease. However, ART requires life-long therapy that is expensive and has the risk of significant side effects. In addition, drug resistance is growing, which requires new and often more costly products. From a patient-centered approach, life-long therapy can be challenging, and unfortunately, stigma and discrimination remain strong throughout the world.
More than 40 percent (40%) of the 36.7 million people who need therapy do not have access to it. More than one million people die from HIV every year, and more than 1.8 million become newly infected. A cure and a preventive or therapeutic vaccine could transform the lives of millions of people.
RENB-HV-12: Preventive and Therapeutic Vaccines
Allogeneic Cell Therapy Platform
Boosting a person’s immune system through vaccination can lead to protection from HIV infection in people who are not living with HIV. In persons living with HIV who are controlling the spread of virus with antiretroviral (ARV) treatment, boosting the immune system in a different way than the virus already has through infection, could allow control of HIV after stopping ARVs.
Renovaro BioSciences’ technology uses the powerful induction of an immune response created by cells from another person to potentially induce such a response. Based on promising in vitro results, a study in non-human primates was begun by the renowned Fred Hutchinson Cancer Research Center, Seattle, Washington.
Preliminary results are expected by late 2023. If successful, human studies potentially could begin in late 2024.

RENB-HV01: Autologous Transplant with Genetically Modified Cells
FDA INTERACT Meeting Held February 2020: It has been proven that gene-editing to knockdown the expression of CCR5 – a door HIV needs to enter and kill CD4+ T cells – in autologous human stem cells (HSC) combined with transplantation can lead to a cure of HIV. However, the approaches currently available require an expensive and risky ablation of the immune system. Even with that drastic intervention, an insufficient number of gene-modified cells survive to achieve durable control of HIV.
We have pioneered a novel enabling technology (ALDH gene modification) that we believe will allow sufficient engraftment of the CCR5 gene-modified HSC to eliminate the need for Antiretroviral Treatment (ART.) Although in vitro and in vivo studies have demonstrated promising results, further development of RENB-HV01 at this time was deemed costly and a long-term undertaking.
Therefore, a business decision was made to sub-license the ALDH gene modification. RENB-HV01 was sub-licensed to Caring Cross with a profit share. Caring Cross is developing CAR-T approach that they believe when combined with Renovaro Biosciences ALDH gene modification, could enhance engraftment of their CAR-T cell therapy.
RENB-HV21: Immunotherapy with Allogeneic NK/GDT cells
Allogeneic Cell Therapy Platform
We are also exploring RENB-HV21, an innovative treatment for HIV with allogenic Natural Killer (NK) and Gamma Delta T-Cells (GDT). It is believed that the GDT cells, a small subset of immune cells that can be infected with HIV, could both be infected by, and be a key factor in controlling the virus. The initial scientific findings were presented during the ASCGT Conference 2021. Renovaro BioSciences has an exclusive license to use the underlying patent to develop RENB-HV-21 for potential treatment or cure of HIV. A successful investigator-initiated Pre-IND was completed in October 2021. However, due to a shift in priorities to the Oncology pipeline, Renovaro BioSciences does not plan to pursue the IND and potential clinical trial in the near to medium-term.


HBV:
RENB-HB01: HBV Gene Therapy
RENB-HB-01 is in early pre-clinical phase as we explore various approaches for gene therapy design elements. If those explorations are successful, it is possible we could begin the regulatory process at the earliest in the first half of 2024. However, our highest priority for investment is the oncology platform, beginning with pancreatic cancer.
Advisors & Collaborators
Strong expertise in the field of cell and gene therapy, HIV and immuno-oncology focusing on developing transformative, multi-indication, platform drugs to change the standard of care of Oncology, HIV and HBV.

Information Request Form
Your inquiries matter. Feel free to use the form below to get in touch, and our team will make it a priority to respond as quickly as possible.
News
Stay informed with the latest updates and announcements in our News Section.
June 14, 2024
Renovaro, Inc. Announces $10 million in Equity Committed
“We appreciate confidence in the Company demonstrated by the investors,” said the Hon. Mark Dybul, MD, CEO.

May 24, 2024
Renovaro Inc. and Amsterdam UMC Cancer Center Poised to Advance Cancer Immunotherapy
This collaboration would bring together Renovaro’s proprietary cancer vaccine technology and the Cancer Center’s expertise in several ancillary immunomodulatory technologies, towards innovative advancements in cancer treatment.

April 30, 2024
Transforming Cancer Detection: RenovaroCube Introduces Flamingo, a novel AI model based on Fragmentomics

April 29, 2024
Avram Miller Talks About Renovaro And Its Impact In Diagnosing Cancers and Infectious Diseases With AI
The potential impact of AI on healthcare is vast and transformative. With the use of AI platforms for diagnosing diseases like cancer, we can expect improved accuracy, efficiency, and outcomes in patient care. Renovaro stands at the forefront of this innovative shift towards a more intelligent healthcare system powered by artificial intelligence.